Beijing Ditan Hospital Xing Huichun/Wang Xi Team at Beijing Ditan Hospital Elucidates Novel Anti-HCC Mechanism of Gut Microbial Metabolites
A recent study by Professor Xing Huichun and Professor Wang Xi's team from Beijing Ditan Hospital, Capital Medical University was published in the international journal Gut Microbes under the title "Gut microbial metabolite butyrate suppresses hepatocellular carcinoma growth via CXCL11-dependent enhancement of natural killer cell infiltration." The research elucidates the immunoregulatory mechanism by which the gut microbiota metabolite butyrate exerts anti-liver cancer effects by enhancing NK cell migration and effector function in both in vitro and in vivo models. These findings provide novel insights into short-chain fatty acid-based immunotherapy for hepatocellular carcinoma and demonstrate promising clinical translation potential. Dr. Zhang Menghan and Dr. Huang Xuefeng from Beijing Ditan Hospital, along with Dr. Zhang Yanlong from Shanxi Bethune Hospital (jointly trained at Beijing Ditan Hospital), served as co-first authors, while Professor Xing Huichun and Professor Wang Xi were corresponding authors.

Hepatocellular carcinoma (HCC) ranks as the sixth most prevalent malignant tumor worldwide and the third leading cause of cancer-related deaths, with continuously rising incidence rates and limited treatment options. Its pathogenesis involves liver injury and fibrosis resulting from chronic liver diseases (such as hepatitis and cirrhosis), as well as dysfunctional immune cells within the tumor microenvironment, collectively promoting tumor progression and metastasis. In recent years, gut microbiota and their metabolites—short-chain fatty acids (SCFAs, including acetate, propionate, and butyrate)—have emerged as crucial extrahepatic regulatory factors that can mitigate inflammation, maintain gut homeostasis, and modulate anti-tumor immune responses. However, the precise mechanisms of SCFAs in HCC remain unclear. Existing studies suggest that butyrate may influence gene expression and immune cell function through epigenetic pathways, indicating that histone modifications and enhancer remodeling could be key to its anti-cancer activity. Building on this evidence, the current study investigates how butyrate suppresses HCC growth by reshaping the tumor immune microenvironment and enhancing NK cell-mediated anti-tumor immunity.
The research team found that HCC patients exhibited significantly reduced gut microbiota α- and β-diversity, with increased pathogenic bacteria (e.g., Escherichia-Shigella, Klebsiella) and decreased butyrate-producing probiotics (e.g., Ruminococcus, Butyricicoccus), along with altered plasma SCFA profiles showing particularly reduced butyrate levels that negatively correlated with clinical parameters and served as an independent prognostic factor for PFS. In mouse tumor models, oral butyrate administration significantly inhibited tumor growth and Ki67 expression while specifically enhancing NK cell infiltration in tumor tissues without major effects on other immune cells, with in vitro experiments further confirming butyrate's ability to directly boost NK cell CD107a degranulation, IFN-γ secretion, proliferation, and cytotoxicity. Mechanistic studies revealed that butyrate upregulated CXCL11 mRNA and protein levels in HepG2/Huh7 cells without affecting their viability, inducing NK cell chemotaxis through the CXCL11/CXCR3 pathway, while integrated ATAC-seq and ChIP-seq analyses demonstrated that butyrate remodeled chromatin accessibility at CXCL11 enhancers by increasing H3K27ac/H3K9ac modifications and recruiting STAT4 to activate transcription, collectively showing for the first time that butyrate epigenetically regulates the CXCL11-NK cell axis to reshape the tumor immune microenvironment and activate anti-HCC immunity, offering new insights for probiotic- or SCFA-based adjuvant therapies.
Butyrate epigenetically remodels the CXCL11 enhancer and recruits STAT4 binding, thereby activating the CXCL11-CXCR3 signaling axis to significantly enhance NK cell tumor infiltration and suppress hepatocellular carcinoma growth. These findings reveal the crucial role of gut microbiota metabolites in activating anti-tumor immunity and suggest potential new strategies for probiotic- or SCFA-based immunotherapy in HCC.
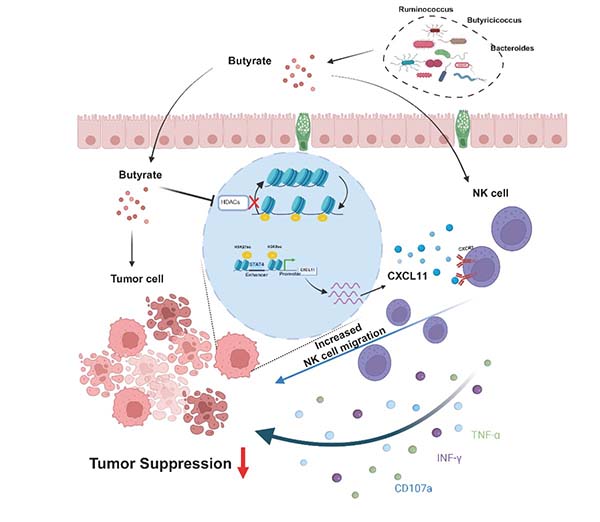
Figure. Butyrate epigenetically remodels the CXCL11 enhancer and recruits STAT4, activating the CXCL11–CXCR3 signaling axis to promote NK cell infiltration into tumor tissue and suppress hepatocellular carcinoma growth (schematic diagram).
This study was supported by grants from the National Key Research and Development Program of China (2022YFC2304500, 2021YFC2301801), the Ministry of Science and Technology (2023YFC2306003), the Beijing Municipal Science and Technology Major Project (Z221100007422002), the Capital Health Development Research Fund (CFH-2024-1-2181), the Beijing iGandan Public Welfare Foundation (iGandanF1082023-GSH011), the National Natural Science Foundation of China (32270635), the Beijing Natural Science Foundation (7232082), and the Beijing Research Center for Respiratory Infectious Diseases (BJRID2024-010).

Xing Huichun is a professor, chief physician, and doctoral supervisor at the Liver Disease Center of Beijing Ditan Hospital, Capital Medical University, specializing in the pathogenesis, diagnosis, and treatment of viral hepatitis, alcoholic liver disease, and other chronic liver diseases. She holds key academic positions including Director of the Microecology Committee at the Beijing Preventive Medicine Association and Vice Chair of the Hepatobiliary Diseases Branch of the China International Exchange and Promotive Association for Medical and Health Care, while also serving on the editorial boards of several leading medical journals such as the Chinese Journal of Hepatology, Journal of Clinical Hepatology, and Chinese Journal of Microecology. With over 40 SCI-indexed publications as first or corresponding author, she has made significant contributions to liver disease research and has authored/co-edited medical textbooks including Case Analysis of Complex Infectious Liver Diseases at Beijing Ditan Hospital, Capital Medical University.

Wang Xi is a researcher, professor and doctoral supervisor specializing in tumor immunology and infection immunity, currently serving as the director of the Beijing Institute of Infectious Diseases at Beijing Ditan Hospital, Capital Medical University, and principal investigator at the National Key Laboratory of Infectious Disease Traceability, Early Warning and Intelligent Decision-Making. He holds multiple leadership roles including Vice Director of Capital Medical University's Department of Oncology, Deputy Director of both the Beijing Key Laboratory of Viral Infectious Diseases and the Pediatric Hematology-Oncology Diagnosis and Treatment Research Center, while also chairing the Infectious Diseases Committee of the Beijing Chronic Disease Research Association and serving as standing committee member of the Chinese Medical Association's Medical Cell Biology Society. An accomplished researcher with publications in high-impact journals including PNAS, Cancer Cell, and Journal of Clinical Investigation, he has led numerous major research projects supported by the National Key R&D Program, National Natural Science Foundation of China, and Beijing High-Level Public Health Talent Special Fund.

















































 京公网安备 11010502052111号
京公网安备 11010502052111号