Cell Publication Led by Beijing Ditan Hospital Reveals Cross-Species Transmission Mechanism of Cattle-Derived H5N1 Influenza Virus
A multidisciplinary team from Beijing Ditan Hospital Capital Medical University, Institute of Microbiology Chinese Academy of Sciences (CAS), Beijing Tongren Hospital Capital Medical University, and Beijing Life Science Academy has published a landmark paper in Cell titled “Receptor binding, structure, and tissue tropism of cattle-infecting H5N1 avian influenza virus hemagglutinin.” The study delivers the first comprehensive view of the hemagglutinin (HA) protein from cattle-derived H5N1, detailing its receptor-binding specificity, structural adaptations, and tissue tropism. By clarifying the molecular basis of HA receptor preference, the work reveals why this virus poses a heightened epidemic threat and underscores the urgent need for continuous surveillance. The co-first authors include Professor Hao SONG (Beijing Ditan Hospital/Beijing Institute of Infectious Diseases), Dr. Tianjiao HAO (Beijing Life Science Academy), Dr. Pu HAN (Institute of Microbiology CAS), Haichen WANG (Hebei University), and Dr. Xu ZHANG (Beijing Tongren Hospital/Beijing Institute of Ophthalmology). Corresponding authors are Academician George Fu GAO (Institute of Microbiology CAS), Professor Ningli WANG (Beijing Tongren Hospital & Henan Academy of Innovations in Medical Sciences), Dr. Lei SUN (Department of Pathology, Beijing Ditan Hospital), and Professor Hao SONG.

The research team performed a comprehensive analysis of the HA protein from the first human-infecting cattle-derived H5N1 strain (A/Texas/37/2024). Surface plasmon resonance assays showed that this HA preferentially binds avian α2-3 sialic acid receptors while retaining weak affinity for human α2-6 receptors. Immunohistochemical staining confirmed strong HA binding to bovine lung and mammary tissues—consistent with clinical observations—whereas H1N1 and H3N2 HAs did not. Notably, the bovine-origin HA also bound human conjunctival, tracheal, pulmonary, and mammary tissues, explaining the conjunctivitis seen in the first reported bovine-to-human transmission.
High-resolution cryo-EM structures of HA complexed with α2-3 and α2-6 receptors revealed an unusually widened receptor-binding site enabling dual-receptor engagement. These molecular insights clarify how cattle-derived H5N1 adapts to multiple hosts and underscore the urgent need for ongoing surveillance of this emerging pathogen’s epidemic and pandemic potential.
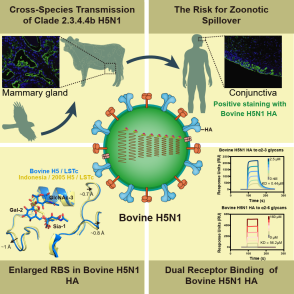
Figure. Cross-species transmission mechanism of cattle-derived H5N1 avian influenza virus
These findings provide molecular-level insights into the cross-species transmission mechanisms of cattle-derived H5N1 viruses. Importantly, they underscore the critical need for sustained surveillance and in-depth research on this emerging pathogen to assess its pandemic potential and inform public health strategies.
This study was supported by grants from the National Natural Science Foundation of China (Project Nos. 92478001 and 82122040)

Professor Hao Song, PhD, is Principal Investigator of the Pathogen Pathogenesis and Immunointervention Research Team at Institute of Infectious Diseases, Beijing Ditan Hospital Capital Medical University/Beijing Institute Infectious of Diseases. He is a recipient of the Young Elite Scientist Sponsorship Program by the China Association for Science and Technology, and the Excellent Member of the Youth Innovation Promotion Association CAS. Dr. Song mainly focuses on the entry and pathogenic mechanism of important enveloped viruses and the discovery of novel viral targets for treatment. Cutting edge tools in biochemistry, cell biology, virology and structural biology were used to study the functions of important proteins like envelope glycoproteins and immune molecules involved in host pathogen interactions and reveal the host jump mechanism and pathogenesis of emerging and re-emerging pathogens. Significant results have been achieved in understanding cross-species transmission of various influenza virus subtypes, entry mechanisms of Chikungunya virus and gammaherpesvirus, and the pathogenesis and prevention targets of Zika virus, SARS-CoV-2, African swine fever virus, and malaria parasites, thereby supporting antiviral drug and vaccine development. He has published more than 60 SCI papers, including Cell and Science (with an H-index of 34). He has led five National Natural Science Foundation of China projects and three sub-tasks of national key R&D programs and co-authored four academic books, The research results were recommended by F1000 Prime, and selected into the Major Progress of Chinese Medicine in 2019. Nine invention patents have been applied for, with five already authorized. He serves as a Member of the Youth Committee, Virology Committee, Chinese Society for Microbiology, and as a Young Editorial Board Member for the hLife journal.

Dr. Lei Sun, PhD, is Associate Professor and graduate supervisor at Beijing Ditan Hospital Capital Medical University, where he is Acting Director of the Department of Pathology and Head of the Pathology Teaching and Research Division. His clinical and research expertise lies in hepatic pathology and the pathological diagnosis of infectious diseases. Dr. Sun is a Committee Member of the 11th Pathology Society of the Beijing Medical Association and is a recipient of Beijing High-Level Public Health Technical Talent and “Youth Programme” Talent of the Beijing Hospitals Authority. As principal investigator, he has led multiple funded projects, including the technology Promotion Program of National Health Commission of the People's Republic of China, the Capital’s Funds for Health Improvement and Research. He has published more than 60 papers in SCI-indexed and Chinese core journals, with 27 as first or corresponding author.

















































 京公网安备 11010502052111号
京公网安备 11010502052111号